So, back from a Medical conference (!) in Germany (so talking all day about medtech. My take away is there isn't much genuine innovation going on outside of AI; AI is increasingly being applied to existing products to differentiate them), but a little puzzled.
Morning before travel; 5.4mmol/l indicated, empty stomach (08:30)
Followed by one of those 200kcal shake breakfasts. At noon, lunch of 2 sardines in oil on toast. 15 minute walk to pick the car up from the garage.
3 hour later; 4.5mmol/l indicated. (15:00)
3 hour after evening meal (think it was a stew), and I snuck a pepperami; 5.7mmol/l indicated (23:00)
Morning of travel; didn't bother with a baseline, its been 5.1-5.5 for a while. Had one of those shakes. Didn't eat until evening so went hungry. Bit of walking in the morning (train, tube, wander a little around Heathrow), not much in Dusseldorf (taxi, hotel, tv)
2 hours after a rump steak, mound of French fries, salad with dressing; 5mmol/l.
Day 2: Morning baseline; 5.8mmol/l.
Breakfast of bacon & eggs, coffee with sugar because I couldn't find the sweetener
Walking around all day, lunch at 3pm, thai curry and rice (!). Probably didn't drink enough
3h later; 5.8mmol/l
Dinner in local pub; Hunter Pork schnitzel and chips. 2h reading confounded me; 4.2mmol/l (11pm)
Skipped any readings yesterday, morning was more conference walking, lunch at 2 with Currywurst, french fries, curry ketchup and chilli cheese (!), no evening dinner, snacked at airport on a packet of crisps. This morning, a predictable 5.6mmol/l before breakfast, and 2 hours after lunch, 5.5mmol/l
Weight is pouring off me.
Currently on 2x500mg Metformin. The doc has given me no direction when to take doses, so have elected for 1 in the morning, 1 in the evening, to flatten out the serum levels.
By now the doc wants me to increase to 3x500 (again, with no further direction), unless "side effects too much". I must have a high tolerance, because right now, the side effects are mild and tolerable.
I am hesitant to further increase the dosage, because the current serum levels are indicative of it "doing its job", ie getting blood sugar levels to within physiological acceptable limits. I'm surprised at the relatively small deltas between high and low (I was expecting to see between 4.5 and 7-8). 2 or 3 hours after eating should have caught somewhere near maxima.
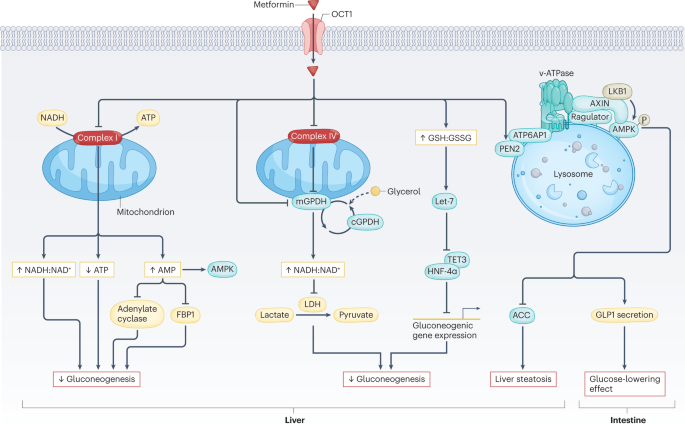
I see that the mechanistic action of metformin is not even barely understood, only hypothesized
This Review highlights the latest advances in our understanding of the mechanisms of action of metformin. Potential repurposing of metformin for other indications is also discussed.

www.nature.com
But then my proverbial eyes rollback when I see this article:
Metformin (dimethylbiguanide) has become the preferred first-line oral blood glucose-lowering agent to manage type 2 diabetes. Its history is linked to Galega officinalis (also known as goat’s rue), a traditional herbal medicine in Europe, found to be rich in guanidine, which, in 1918, was shown...

link.springer.com
100 year history of pharmacological use, and in crude form, perhaps 1000 years of use, and no one really knows how it works.
Kidney function tests;
Urine Microalbumin; 10.5mg/l (well within normal range)
Urine albumin/creatinine ratio; 0.86mg/mmol (bang in the middle of normal)
Urine creatinine level; 12.2 mmol/l (for my age, should be more than 1.3, but less than 26, so again, very normal)
GFR calculated abbreviated MDRD; 76 mL/m/1.73m
The "normal" value varies depending on other measurements. For Kidney Research UK, Over 90, indicates no kidney issue. Between 60 and 90, if no other issues, also normal. However, for the National Institutes of Health, anything over 60 is normal.
So I've no reason to suspect kidney disease. If anything, those kidney function tests might even have gotten "better", now that my morning BP has averaged 119/75 over 19 days, and afternoon 120/74 over the same period (ie. if there was a kidney problem, I'd be overdosing on metformin, with concomitant risk increasing of lactic acidosis). But maybe the lack of high glucose peaks might be associated with not drinking enough (I typically have maybe a 1000mls of liquid a day in addition to meals, so nowhere near the benchmark 2000mls). I've no symptoms associated with lactic acidosis.