Eddy Edson
Well-Known Member
- Relationship to Diabetes
- Type 2
Caught my eye ... a pilot study treating a T2D patient with a clapped-out pancreas with islet tissue grown from his stem cells:
I think there are a few islet transplant plays at clinical trial stage. These guys say of theirs:
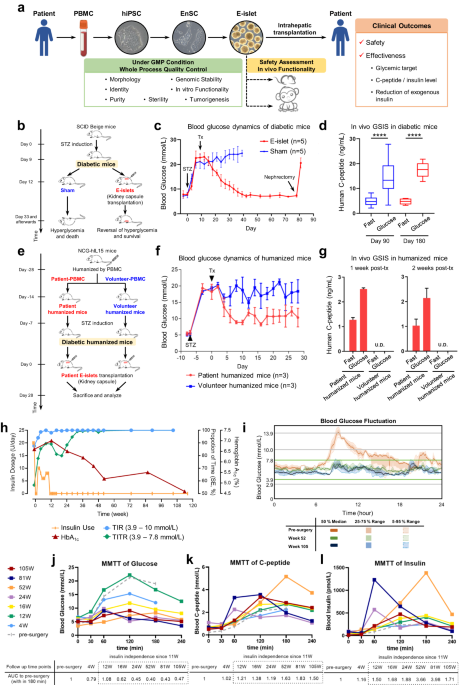
Collectively, we report the first-in-human tissue replacement therapy using autologous E-islets for a T2D patient with impaired islet function. The first 27-month data revealed significant improvements in glycemic control, and provided the first evidence that stem cell-derived islet tissues can rescue islet function in late-stage T2D patients. The grafts were well tolerated with no tumor formation or severe graft-related adverse events.
....
Despite the common proof-of-concept purpose, there are some distinctions among the published trials10,11 and ours. First, [their] EnSC-based islet regeneration system is unique, in that EnSCs are nontumorigenic in vivo12 and amenable for efficient mass production of islets as they are endoderm-specific and developmentally closer to pancreatic lineages. Second, our pilot study chose a T2D rather than T1D patient, which not only precluded the interference from autoimmune conditions for the assessment of engraftment and functionality of E-islets but also extended the scope of indications for islet transplantation. As for the limitations of this study, we cannot completely rule out the possibility that the residual endogenous islets benefitted from the surgery and acquired functional improvements. Therefore, an increase in sample size and additional trials of T1D patients with complete loss of islet β cells will help draw definitive conclusions on the causative role of E-islets in the achievement of glycemic targets.
So, how much time to get this through trials & into the clinic? Dunno, maybe .... 10 years? 🙂
I think there are a few islet transplant plays at clinical trial stage. These guys say of theirs:
Collectively, we report the first-in-human tissue replacement therapy using autologous E-islets for a T2D patient with impaired islet function. The first 27-month data revealed significant improvements in glycemic control, and provided the first evidence that stem cell-derived islet tissues can rescue islet function in late-stage T2D patients. The grafts were well tolerated with no tumor formation or severe graft-related adverse events.
....
Despite the common proof-of-concept purpose, there are some distinctions among the published trials10,11 and ours. First, [their] EnSC-based islet regeneration system is unique, in that EnSCs are nontumorigenic in vivo12 and amenable for efficient mass production of islets as they are endoderm-specific and developmentally closer to pancreatic lineages. Second, our pilot study chose a T2D rather than T1D patient, which not only precluded the interference from autoimmune conditions for the assessment of engraftment and functionality of E-islets but also extended the scope of indications for islet transplantation. As for the limitations of this study, we cannot completely rule out the possibility that the residual endogenous islets benefitted from the surgery and acquired functional improvements. Therefore, an increase in sample size and additional trials of T1D patients with complete loss of islet β cells will help draw definitive conclusions on the causative role of E-islets in the achievement of glycemic targets.
So, how much time to get this through trials & into the clinic? Dunno, maybe .... 10 years? 🙂