Northerner
Admin (Retired)
- Relationship to Diabetes
- Type 1
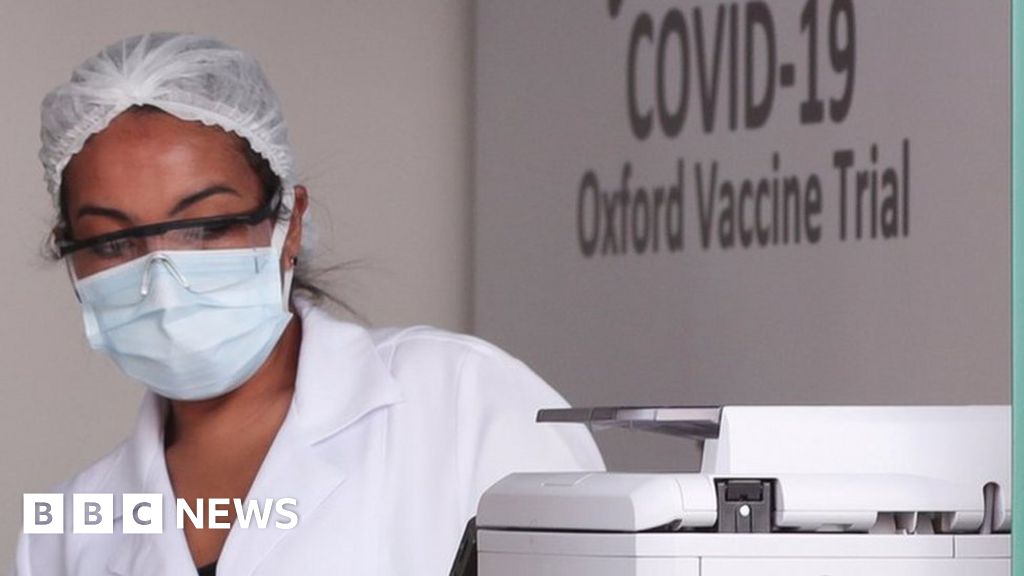
The Brazilian National Health Surveillance Agency (Anvisa) announced today that it is investigating data received on the death of a volunteer in a clinical trial of the COVID-19 vaccine developed by Oxford University and the pharmaceutical company AstraZeneca.
In an email sent to Medscape Medical News, the agency states that it was formally informed of the death on October 19. It has already received data regarding the investigation of the case, which is now being conducted by an international security assessment committee.
The identity of the volunteer and cause of death have not yet been confirmed by any official source linked to the study.
A report in the Brazilian newspaper O Globo, however, says the patient who died is a 28-year-old doctor, recently graduated, who worked on the front line combating COVID-19 in three hospitals in Rio de Janeiro. He reportedly died last Thursday due to complications from COVID-19. It is unknown whether the volunteer received the vaccine or placebo.
 www.medscape.com
www.medscape.com
Very sad
In an email sent to Medscape Medical News, the agency states that it was formally informed of the death on October 19. It has already received data regarding the investigation of the case, which is now being conducted by an international security assessment committee.
The identity of the volunteer and cause of death have not yet been confirmed by any official source linked to the study.
A report in the Brazilian newspaper O Globo, however, says the patient who died is a 28-year-old doctor, recently graduated, who worked on the front line combating COVID-19 in three hospitals in Rio de Janeiro. He reportedly died last Thursday due to complications from COVID-19. It is unknown whether the volunteer received the vaccine or placebo.

Brazil Confirms Death of Volunteer in COVID-19 Vaccine Trial
It is not yet known whether the death is related to the vaccine developed by the University of Oxford and AstraZeneca.
Very sad